Who is a member?
Our members are the local governments of Massachusetts and their elected and appointed leadership.
 The Baker-Polito administration today announced allocation and distribution plans for the first round of COVID-19 vaccine shipments to Massachusetts, set to begin around Dec. 15.
The Baker-Polito administration today announced allocation and distribution plans for the first round of COVID-19 vaccine shipments to Massachusetts, set to begin around Dec. 15.
According to the administration, the state’s first shipment of nearly 60,000 doses of the Pfizer vaccine was ordered from the federal government on Dec. 4 and will be delivered directly to 21 hospitals in eight counties, as well as to the Department of Public Health’s Immunization Lab. Doses will then be redistributed for access to 74 hospitals in all 14 counties for front line medical workers.
The next 40,000 doses of Pfizer vaccine will be allocated to the Federal Pharmacy Program to begin vaccinating staff and residents of skilled nursing facilities, rest homes and assisted living residences. The vaccine is being prioritized for these groups to maximize life preservation and to support the health care system, the administration announced.
Based on information available at this time, Massachusetts is expecting 300,000 first doses of the vaccine to be delivered by the end of December. The first vaccines, manufactured by Moderna and Pfizer, will require two doses administered three to four weeks apart.
The administration has launched a new vaccine website, and has posted a vaccine presentation. Answers to Frequently Asked Questions are also available online.
While all delivery dates and quantities are subject to change due to ongoing federal approval and allocation, the administration plans to receive and distribute more than 2 million doses to priority population groups by the end of March.
In collaboration with the COVID-19 Vaccine Advisory Group, the administration designated groups of medical workers, first responders and residents most at risk for serious illness to receive the vaccine before the general population. The Vaccine Advisory Group is made up of medical, infectious disease and public health experts as well as representatives from communities of color and representatives of high-risk populations.
The administration noted that communities of color and at-risk populations are prioritized throughout the process to maximize life preservation and to prevent serious complications from COVID related illnesses.
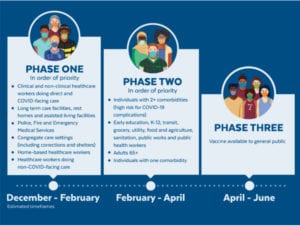
Anticipated Vaccination Phases and Timeline
Phase 1 (December 2020-February 2021, in order of priority):
• Clinical and non-clinical health care workers doing direct and COVID-facing care
• Long-term care facilities, rest homes and assisted living facilities
• Police, fire and emergency medical services
• Congregate care settings (including shelters and corrections)
• Home-based health care workers
• Health care workers doing non-COVID facing care
Phase 2 (February 2021-April 2021, in order of priority):
• Individuals with two or more comorbidities (high risk for COVID-19 complications)
• Early education, K-12, transit, grocery, utility, food and agriculture, sanitation, public works and public health workers
• Adults aged 65 and up
• Individuals with one comorbidity
Vaccines will become available to the general public beginning in April 2021.
The first shipments of the vaccine are expected to contain doses manufactured by Pfizer and Moderna. While both Pfizer and Moderna vaccines are pending FDA emergency use authorization, Massachusetts will not distribute the COVID-19 vaccine until it receives this authorization.
The infectious disease experts in the state’s academic medical centers have pledged to review the EUA data and provide an independent opinion about their safety and efficacy.